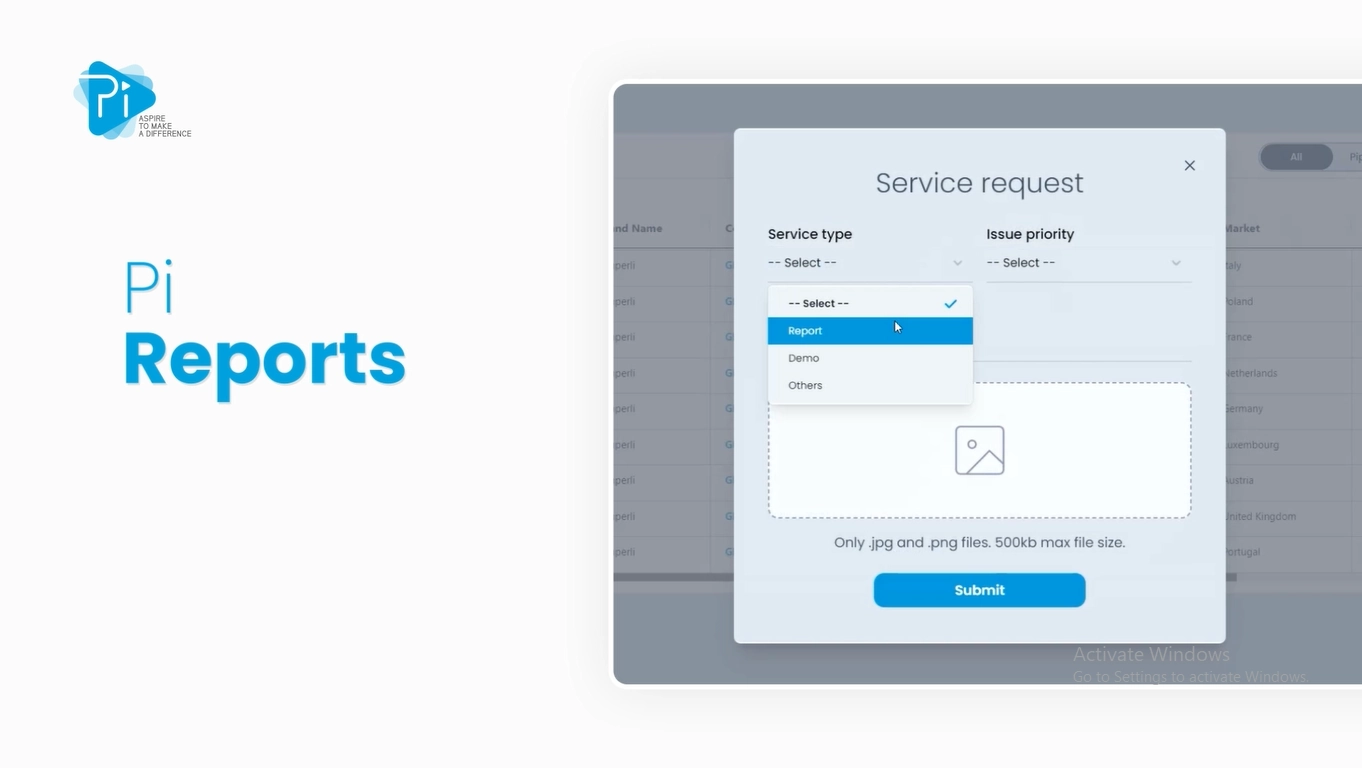
Pi Reports
Dig Deeper With Our Customized Reports
Pi Pharma Intelligence takes the necessary steps and analysis to provide service reports to identify market access barriers, analyse regulatory and patent trends in the therapy areas and overview all clinical programs around a particular molecule
Dig Deeper With Our Customized Reports

Best-In-Class Modules
-
Recommendation on FTO and access strategy
- Using this analysis, you will learn about patent constraints in the region and their expiration dates. It suggests the date of earliest commercialization and the model, which may be local production, secondary packaging, or importation of finished products.
-
Clinical overview
- The report compiles information about all the clinical trials around the molecule that could lead to new market entry, either in dosage forms or new indications.
-
Risk assessment
- Using this report, you can anticipate risks to your portfolio from new entries and treatments.
-
Wishlist
- Follow up on latest updates and status changes on a list of molecules that you wish to track
Reports That Tell The Whole Story

Reports That Tell The Whole Story
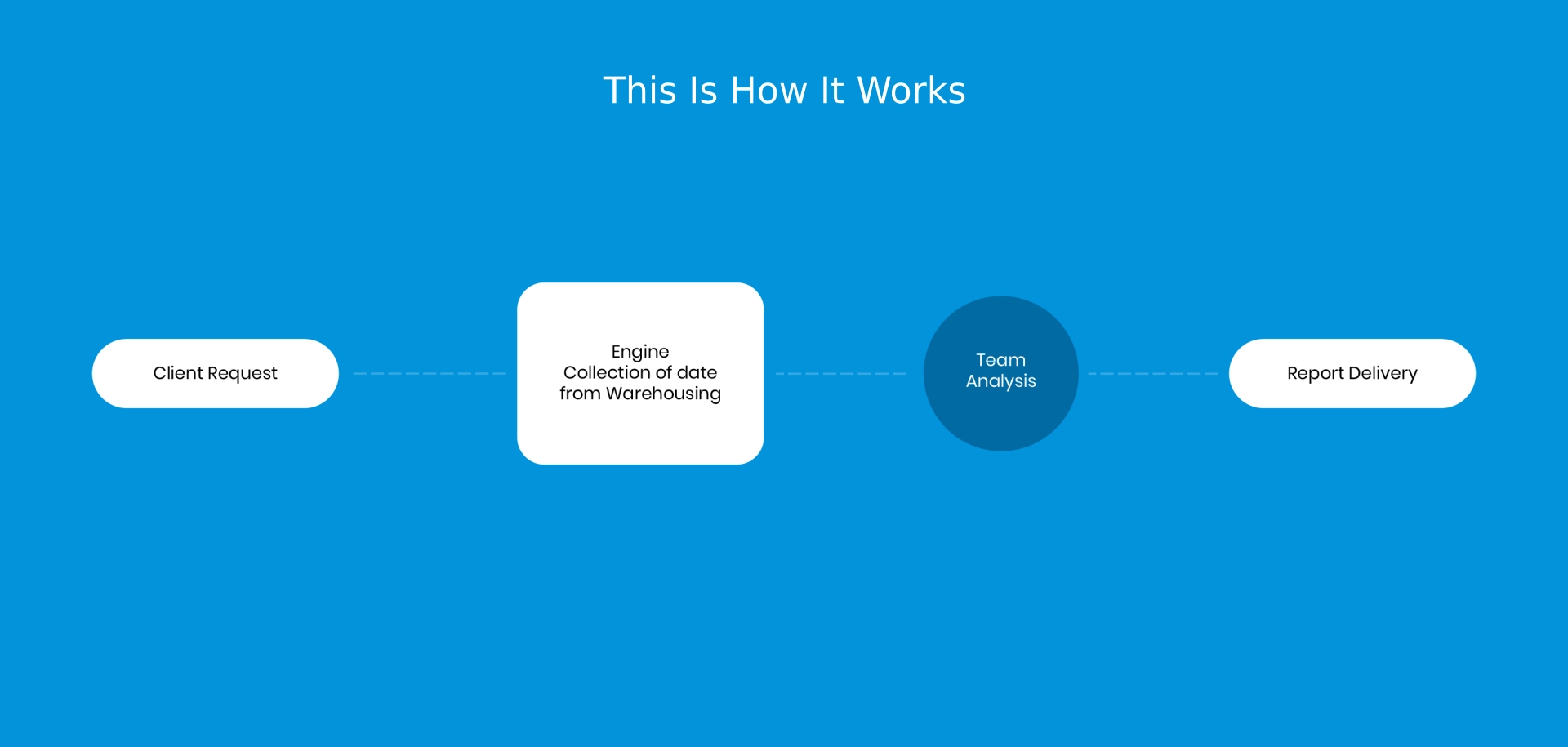
Pi Pharma intelligence, through its service reports, provide :
- An assessment evaluating a generic entry into the market.
- Evaluation of the patent situation
- Identifying possible generic market access options
- Provide generic suppliers, including evaluation of potential generic FDF/ APIs suppliers.
Success Stories
Tell Us How We Can Help You
-
Are you wondering about the best market access strategy?
-
We advise you on sourcing countries and the best model in the market to ensure the earliest commercialization date.
-
Are you looking for a finished dosage form supplier?
-
Our platform technology allows us to identify FDF suppliers With their development stage.
-
Are you looking for API suppliers?
-
Our platform technology allows you to identify API suppliers who are EU and US DMF-approved.
-
Do you want to know which therapy area will face the most patent cliffs?
-
With our database, we can determine when a molecule's basic patent will expire.
-
Are you curious about clinical updates on specific molecules?
-
Our clinical reports will analyze each molecule's clinical programs and classify them based on being head-to-head, primary drug and many other features.
-
Do you want to evaluate the risk in your portfolio?
-
Our dataset can assess the risks on prices and the number of upcoming generics that can compete with you.
Did We Get Your Interest ?
If you're interested in learning more about how our service reports can help you, you can book a demo.